
Thomson's Plum Pudding model

Thomson's conclusion that the corpuscles were present in all kinds of matter

He received the Nobel Prize

The models of the Thomson's atom and Rutheford's atom.
the mass of the hydrogen atom. Thomson's Atomic Model

building an atom model. He also showed that electrons can move from a lower

Fun visualisation of the first atom model to contain subatomic particles.

Characteristic of the classic atomic models (Dalton, Thomson, Rutherford and

Atomic Model/ Theory Timeline

B: Thomson's Model

Electrons in Rutherford's atom could neither be at rest nor in motion.

The Rutherford atomic model has been alternatively called the nuclear atom,

Model of the Atom according to J.J. Thompson

Posted in Atomic Structure, Matter & Properties
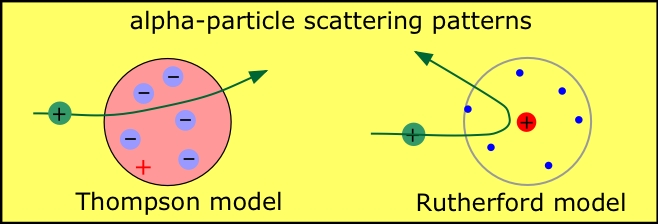
thompson and rutherford models of the atom. Even though the planetary model

Thomson's model is a "plum-pudding" model. He pictured the small electrons

1937. atom-bohr.gif

Rutherford s atomic model. (i) Every atom consists of a nucleus containing

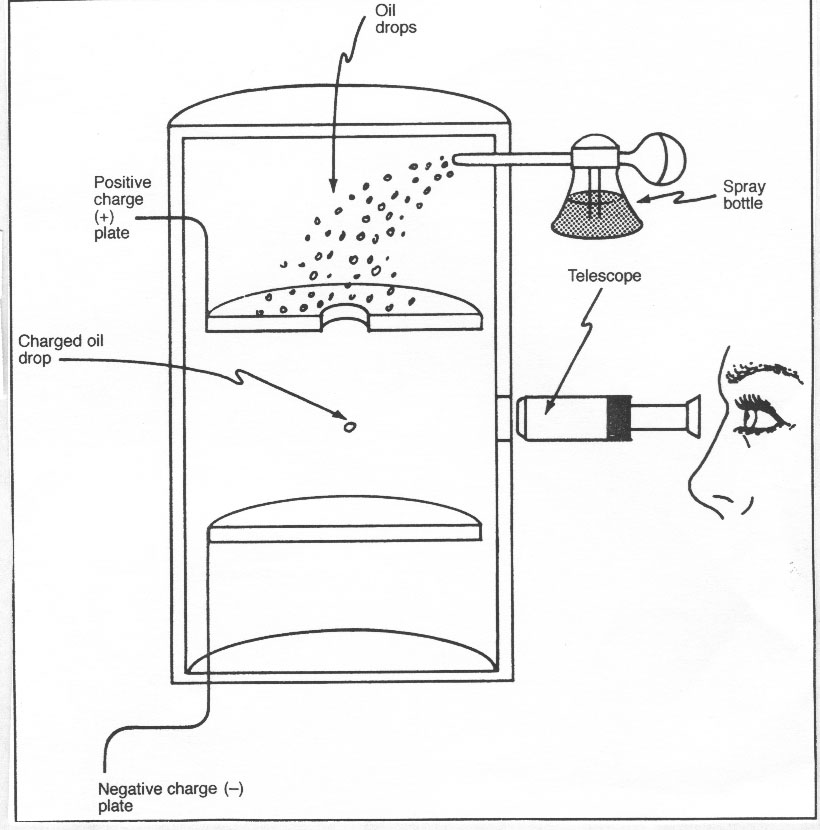
jj thomson experiments atomic theory experiments

Raisin Pudding Model Rutherford's Atomic Model
No comments:
Post a Comment